説明
購入する Zovirax 日本のボディビルショップで、世界中に配送します。クレジットカードのご利用が可能です。 MasterCard, Visa, AmEx およびPayPal決済が可能です。
テスト ハイクオリティ Acyclovir オンライン販売. Generic’s Zovirax Cream が最高品質 Acyclovir 5% cream tube (Zovirax) PCTの有名プロデューサーから
私たちのオンライン薬局は、世界中にPCTをお届けしていますので、あなたがどこに住んでいても、あなたの注文を速く、安全に、慎重にお届けすることができます。: アメリカ、カナダ、イギリス、アイルランド、スペイン、ドイツ、フランス、イタリア、オランダ、南アフリカ、デンマーク、スウェーデン、フィンランド、ノルウェー、日本、ニュージーランド、その他. また、紛失、押収、破損した小包の再発送も承っております。
アメリカ、カナダ、イギリス、アイルランド、スペイン、ドイツ、フランス、イタリア、オランダ、南アフリカ、デンマーク、スウェーデン、フィンランド、ノルウェー、日本、ニュージーランド、その他. また、紛失、押収、破損した小包の再発送も承っております。 ステロイド販売 を、お客さまに安心してお使いいただけます。
私たちのフレンドリーなスタッフがいつでもお手伝いします。 すべての荷物にトラッキングコードがついています. ご注文の状況をメールでお知らせするほか、専用のオンラインサービスで現在の状況を常に確認することができます。
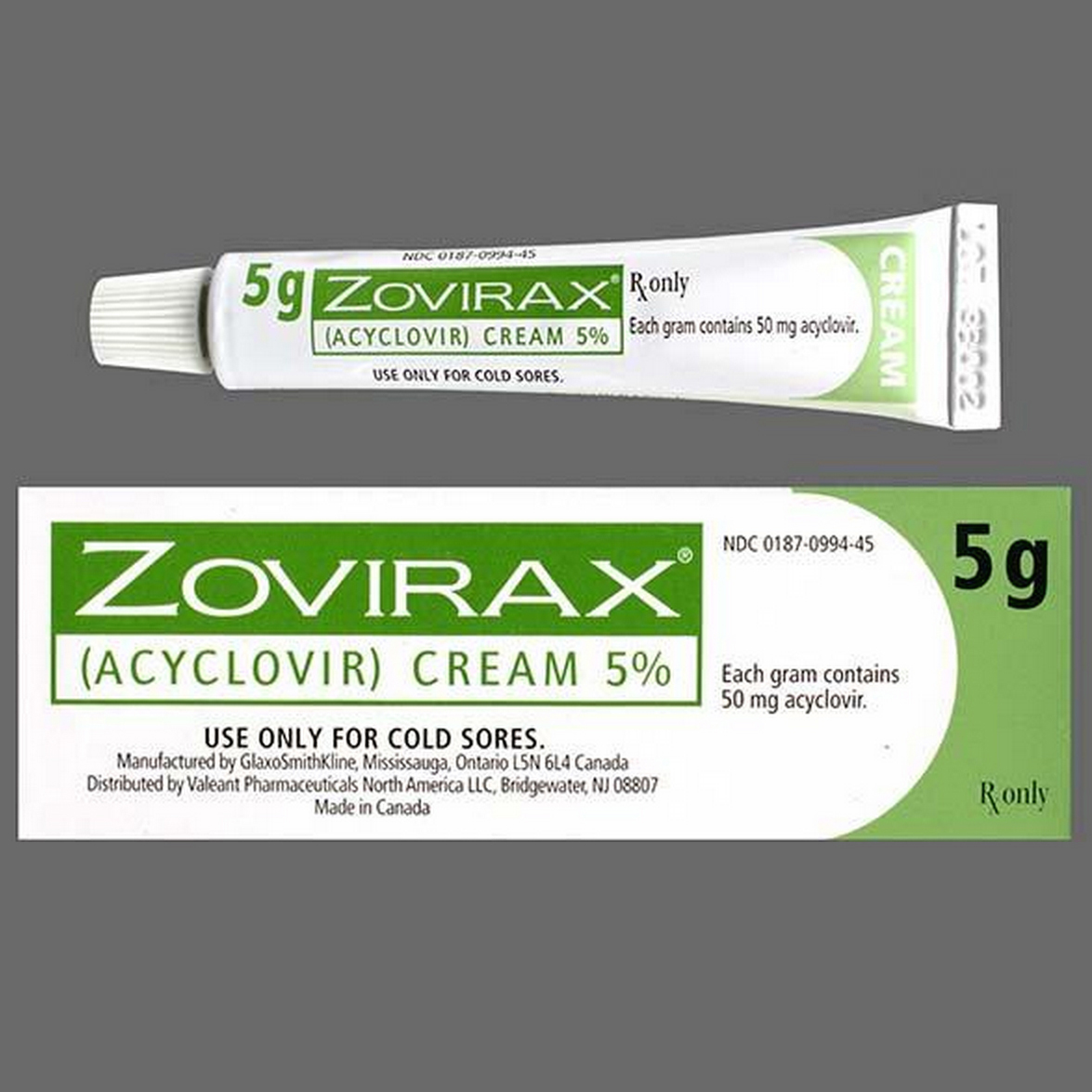
Zovirax Cream というのが広く知られている Zovirax, Acyclovir Cream
の他の別称。 Zovirax Cream (Acyclovir): Generic, Acyclovir, Zovirax Cream, Acyclovir, Zovirax Cream, Zovirax 5% tube, Zovirax Cream 5% tube.
これらはすべて、同じ活性物質の異なるブランドである – Acyclovir
Indications and Usage for Zovirax Cream
Zovirax Cream (Acyclovir Cream) is a herpes simplex virus (HSV) nucleoside analogue DNA polymerase inhibitor indicated for the treatment of recurrent herpes labialis (cold sores) in immunocompetent adults and adolescents 12 years of age and older.
Zovirax Cream Dosage and Administration
Zovirax Cream should be applied five times per day for four days. Therapy should be initiated as early as possible following the onset of signs or symptoms of herpes labialis i.e., during the prodrome or when lesions appear.
For adolescents 12 years of age and older, the dosage is the same as in adults.
Dosage フォームs and Strengths
Each gram of Zovirax Cream, 5% contains 50mg of acyclovir.
Warnings and Precautions
General
Zovirax Cream should only be applied on the affected external aspects of the lips and face in patients with herpes labialis. Because no data are available, application to human mucous membranes is not recommended. Zovirax Cream is intended for cutaneous use only and should not be used in the eye or inside the mouth or nose.
Contact Sensitization
Zovirax Cream has a potential for irritation and contact sensitization.
The effect of Zovirax Cream has not been established in immunocompromised patients.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in practice.
In five double-blind, placebo-controlled trials, 1,124 patients were treated with Zovirax Cream and 1,161 with placebo cream. Local application site reactions were reported by 5% of patients receiving Zovirax Cream and 4% of patients receiving placebo. The most common adverse reactions at the site of topical application were dry lips, desquamation, dryness of skin, cracked lips, burning skin, pruritus, flakiness of skin, and stinging on skin; each adverse reaction occurred in less than 1% of patients receiving Zovirax Cream and placebo. Three patients on Zovirax Cream and one patient on placebo discontinued treatment due to an adverse event.
An additional study, enrolling 22 healthy adults, was conducted to evaluate the dermal tolerance of Zovirax Cream compared with vehicle using single occluded and semi-occluded patch testing methodology. Both Zovirax Cream and placebo showed a high and cumulative irritation potential. Another study, enrolling 251 healthy adults, was conducted to evaluate the contact sensitization potential of Zovirax Cream using repeat insult patch testing methodology. Of 202 evaluable subjects, possible cutaneous sensitization reactions were observed in the same 4 (2%) subjects with both Zovirax Cream and placebo, and these reactions to both Zovirax Cream and placebo were confirmed in 3 subjects upon rechallenge. The sensitizing ingredient(s) has not been identified.
The safety profile in patients 12 to 17 years of age was similar to that observed in adults.
Postmarketing Experience
In addition to adverse events reported from clinical trials, the following events have been identified during post-approval use of acyclovir cream. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to acyclovir cream.
General: Angioedema, anaphylaxis.
Skin: Contact dermatitis, eczema.